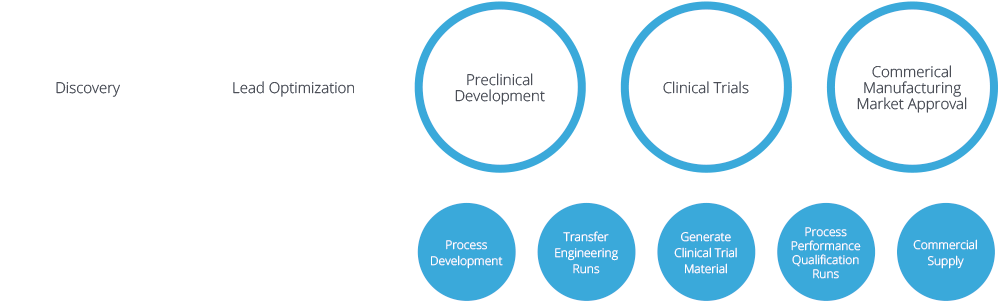
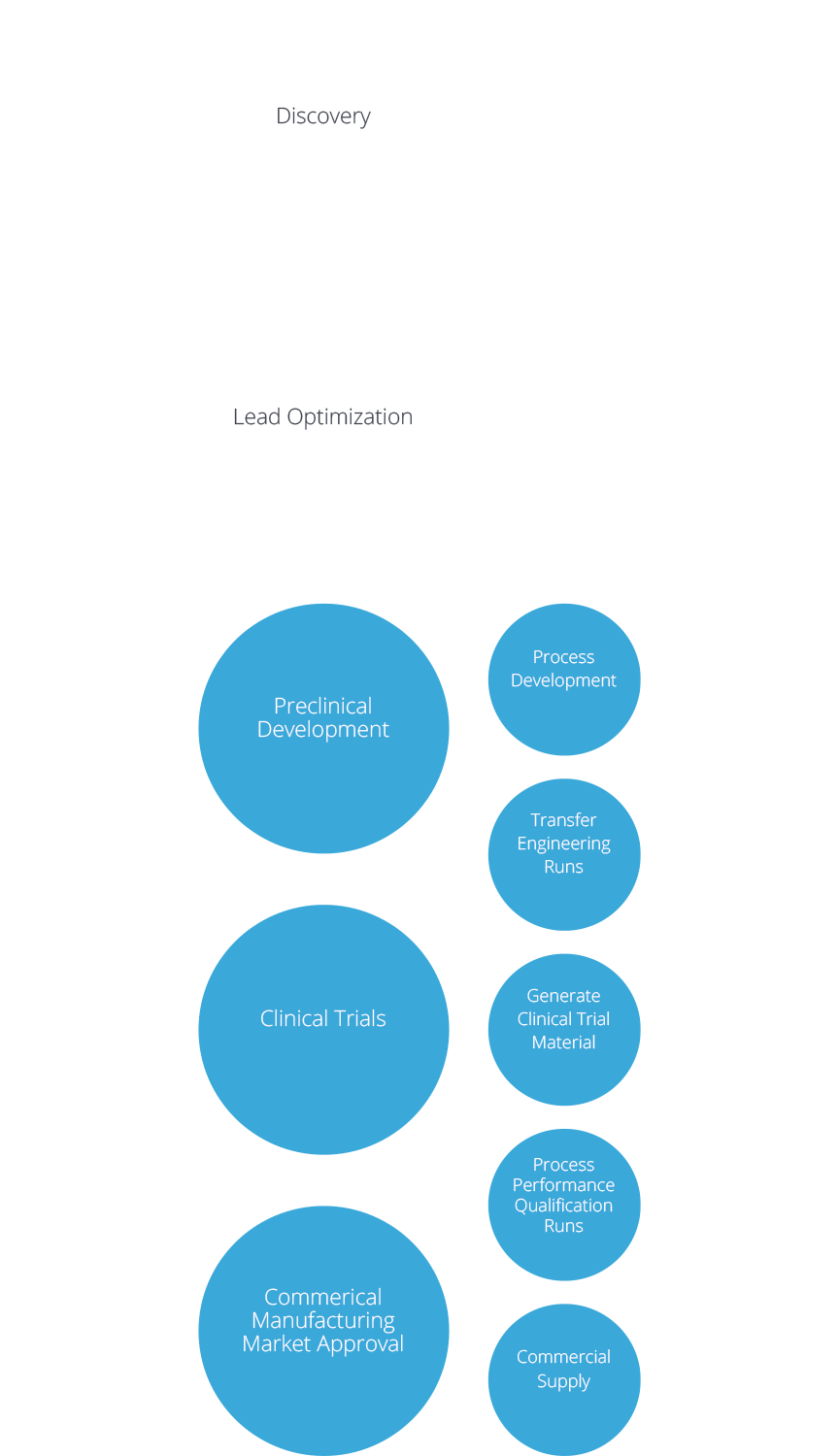
CDMO services at the company site in Germany offer the whole value chain from development through clinical phase I-III to full commercial manufacturing of drug substance and drug product, quality control, packaging and storage. This applies to live and inactivated viral vaccines up to BSL2 and the filling of monoclonal antibodies and therapeutic proteins in vials and pre-filled syringes.
Following CTM production at our US site in Rockville, MA, products are being transferred to Germany for commercial manufacturing. IDT Biologika shows a a high flexibility to respond to customer requests by adapting technologies and processes and ensuring fast processing of orders.






Am Pharmapark, 06861 Dessau-Rosslau, Germany
Phone: +49 (0)34901 8850
E-Mail: info@idt-biologika.com
Germany, Dessau-Roßlau
Germany, Magdeburg