IDT Biologika offers process development and GMP compliant manufacturing of Viral Vaccines, Oncolytic Viruses, Gene and Immunotherapeutics as well as Biologics in its facilities in Germany and US.
With decades of experience in the commercial production of live viral BSL2 vaccines and fill/finish of biologics at our FDA and EMA approved sites (ANVISA ready), IDT Biologika handles every step in process development, manufacturing, quality control and packaging processes. We strive for a strong collaboration with our client partners to support the development of their vaccines and other biopharmaceuticals.
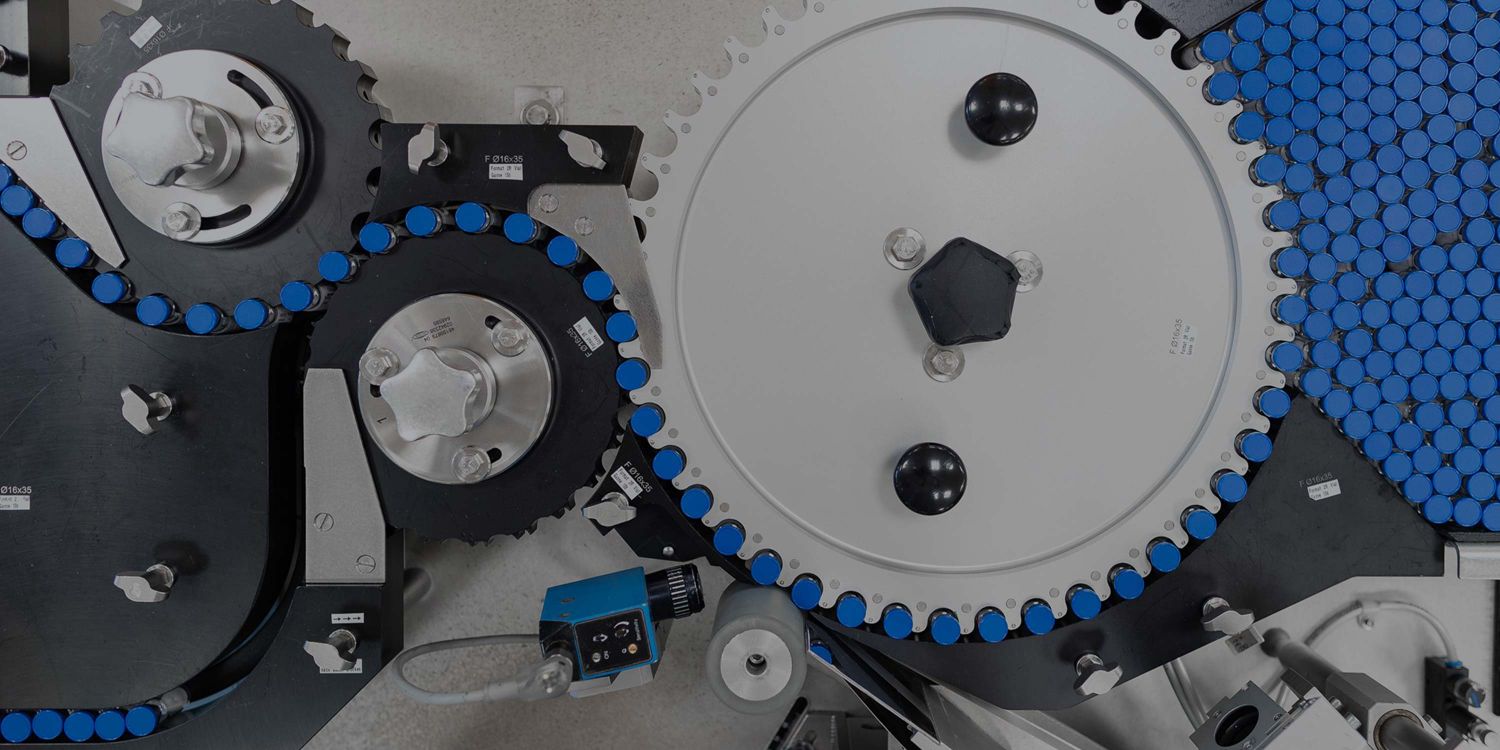
Cell Line Development
Transfer of Customer Technologies
Viral Drug Substance Upstream and Downstream Development
Formulation Development
Stability Evaluation
Process Development for Drug Product Manufacturing
Manufacturing of Non GMP Material

Process Transfer
Aseptic Process Validation
Cell and Virus Banking
Drug Substance Manufacturing using different Technologies

Process Transfer
Compounding / Bulk Formulation
Aseptic filling
Lyophilization
Automated, semi-automated and manual visual Inspection
Process Validation

Method Transfer
Specific Method Development
Method Implementation and Validation
Raw Material Testing and Release
In Process Testing
Release Testing
Stability Testing
Environmental Monitoring
Utility Monitoring
Cleaning Validation Analytics
Methods: Microbiological testing, Molecular biology testing, Biological testing, Chemical-physical testing

Vials and Pre-filled Syringes
Labelling, Blistering, Packaging
Safety Device Assembly, Labelling, Packaging
Pen/Autoinjector Assembly and Packaging
Combination Products
Serialization (track and Trace)
Aggregation
Hand Packaging of Clinical Samples
Temperature controlled storage between -65 and 25°C (-85 and 77 °F)
Stock control via SAP